When I only have this kind of picture to play, you should know that it is not easy.
you may be able to judge how dangerous and cool a chemical /reaction is by video on the Internet. Dangerous and cool experiments, such as aluminothermic reactions, can always find a lot of popular videos online with the words "do not imitate".
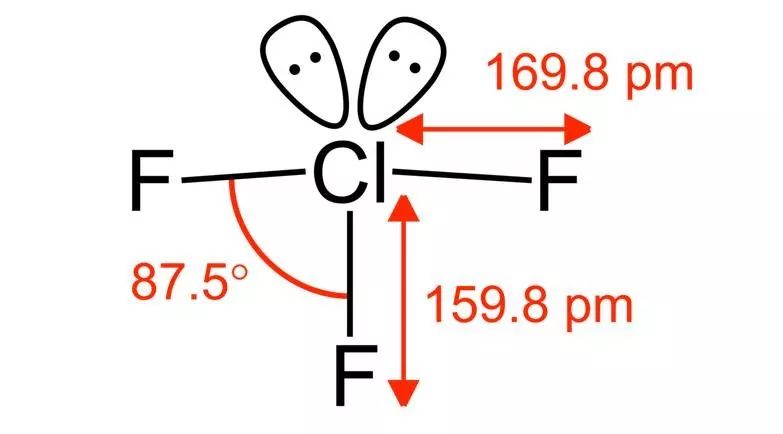
and this is really just average. You won't easily see something that is really scary to the legendary level-because no one wants to touch it, and that's what we're going to see today: chlorine trifluoride (ClF3). Rummaging through the whole oil pipe, we can only find a live video related to it. The person who took this video can be said to be a real warrior (youtube ID:blogrpnet, all right, I believe you), and the whole content of this video can be described as burning, frying, burning.
the French tags that appear in this video are:
plexiglas: plexiglass
Gants de caoutchouc: rubber gloves
cuir propre: clean leather
cuir sale: dirty leather
Selecting from turquoise formal attire to perfectly illustrate the essence of fashion. Just check our these selections on your leisure.
masque: protective mask
bois: Wood
Gant + eau: gloves + water
this is because things like chlorine trifluoride are so oxidizing. It simply catches who reacts with whom, even sand and concrete, which are very fire-resistant in common sense, are even more difficult to deal with than fluorine. In the face of such an out-of-control reaction, the formation of a big hydrofluoric acid fog seems to have become less important.
and the scariest thing is that people have thought of using it on a large scale in history (crazy! ). Germany tried to turn it into an incendiary weapon during World War II, and the Americans tested it as rocket fuel. Of course, none of these attempts were successful: of course, the product burned strong enough, but it burned so indiscriminately that it hung up before it was launched, and the Nazis couldn't stand it.
and during the period when people tried to test chlorine trifluoride as a rocket propellant, it also left an epic level. ) the leakage accident. Chlorine trifluoride is usually contained in metal containers, which is almost the only way to control it temporarily, as long as it is pre-treated to form a metal fluoride protective layer, it can remain stable in some common metal containers. In this accident, the metal container turned over: according to records, people put the metal container in a dry ice bath to cool it in order to make the chlorine trifluoride more stable at low temperature, but the low temperature also made the metal container wall brittle. As a result, people ushered in a catastrophic scene: 907kg's cold liquid chlorine trifluoride leaked out. The chlorine trifluoride not only burned through the 30-centimeter-thick concrete floor, but also corroded nearly a meter of gravel below. Of course, this process is accompanied by a large amount of toxic steam, including chlorine and hydrogen fluoride. An eyewitness exclaimed: "the concrete is on fire!" (The concrete was on fire!)
(it's a pity that no video material was left behind in this accident)
Rocket engineer John Clark in his "Ignition!" An informal brief history of rocket liquid fuel also left a classic comment on chlorine trifluoride: "in the face of such a problem, the solution I have always suggested is a pair of high-quality running shoes."
well, chemistry is really amazing.
PS: chlorine trifluoride still has a small number of specific applications, which are said to be used to remove oxides from semiconductor surfaces in the semiconductor industry, but you won't see it in life. This video provides a very dangerous demonstration, but no one can get it anyway (you won't want it either! ), so I am not worried about _ (: worry "∠) _ (even Tencent Video is not worried, the video passed the examination quickly.) )
read more: the transcript of the epic spill accident I saw here: http://web.archive.org/web/20060318221608/http://www.airproducts.com/nr/rdonlyres/8479ed55-2170-4651-a3d4-223b2957a9f3/0/safetygram39.pdf